Velsera Appoints Jamie Coffin, PhD, as Chief Executive Officer
Read more
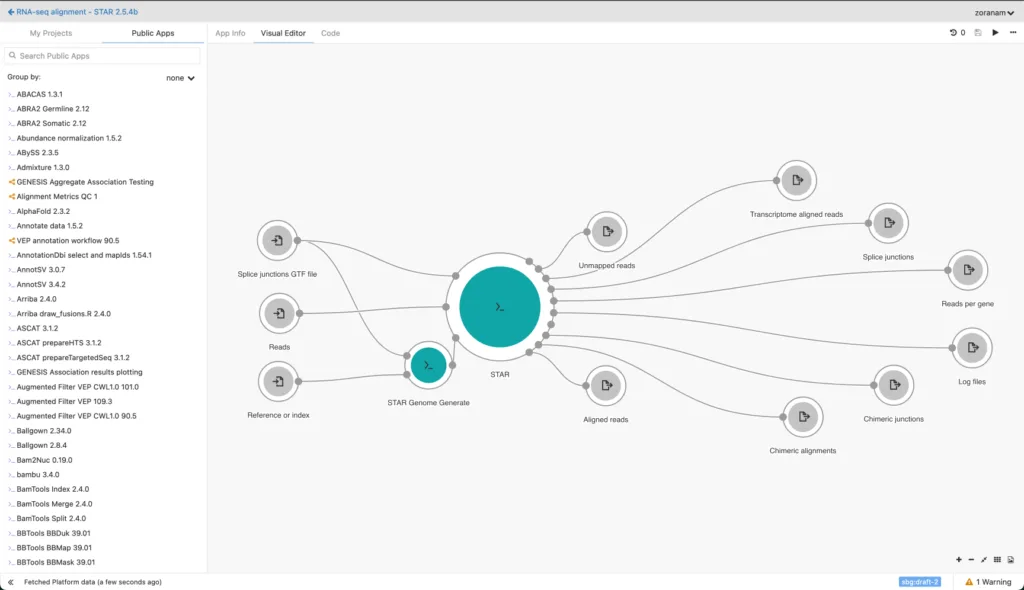
Seven Bridges Platform
Our Trusted Research Environment (TRE) for bioinformatic analysis & pipeline development - Empowering precision medicine by bridging research and clinical diagnostics seamlessly.
The Seven Bridges Platform provides best-in-class, cloud-optimized algorithms along with complete flexibility to bring your proprietary and innovative tools to where the data lives, your teams are empowered to ask the hard questions without doing the heavy lifting, whether in research or clinical diagnostics.
For drug discovery: The Seven Bridges Platform is a scalable and highly secure cloud-based environment that accelerates your team’s ability to leverage biomedical data throughout the drug discovery life cycle, including:
For clinical diagnostics: The Seven Bridges Platform also supports secondary NGS analysis for clinical applications, helping diagnostic labs streamline workflows and deliver reliable, reproducible results. Key use cases include:
Already trusted by 18,000 researchers from 50+ countries running 650k+ analyses per quarter
Why use Seven Bridges
Secure & compliant
Easy & efficient
Seven Bridges makes working on complex, collaborative research projects a breeze:
Secure
Trusted

Uncover new insights into cancer.

Accelerate pediatric research and discovery.

Access leading heart, lung, blood, and sleep data.
Who we help accelerate
Biotech & Pharma
The volume of clinical-genomic data available to researchers is exploding. But without the right tools and advice it can be hard to cut through the noise and find the insights you need. Our best-in-class tools supported by our in-house Bioinformatics and Science teams can help you to do just this and accelerate your time to finding that next big breakthrough.

Non-profits & Government Agencies
Velsera’s Seven Bridges platform is already widely in use across government, academic and commercial research. Our teams of in-house Scientists can help engage this community to make better use of your assets – either to further your research interests, drive new revenue streams, or both!

Clinical Diagnostic Laboratories
Have you got a promising diagnostic test in development? Velsera can partner with you to bring new, promising tests to market by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines on Seven Bridges. Additionally, we can help your future laboratory customers with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

NGS Assay & Equipment Manufacturers
Have you got a promising diagnostic test in development? Or do you have novel equipment that you want to drive uptake of in the clinical setting? Velsera can partner with you to bring new, promising tests to market by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines in Seven Bridges. Additionally, we can help your future laboratory customers with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.
Solutions
Analysis & Bioinformatics
The volume of clinical-genomic data available to researchers is exploding. But without the right tools and advice it can be hard to cut through the noise and find the insights you need. Our best-in-class tools supported by our in-house Bioinformatics and Science teams can help you to do just this and accelerate your time to finding that next big breakthrough.

Clinical & 'omic data unlock
With Velsera’s help, diagnostic labs are uncovering opportunities embedded within the data they already collect. By harmonizing scattered, siloed, and unstructured clinical-genomic information, we are helping labs assemble data and make this available to Biotech and Pharma for novel discovery work. Learn how we can help you access new data sources to advance your research priorities.
NGS Assay Development & Expansion
Has your lab recognized an unmet need in the market? Velsera helps ambitious diagnostic labs generate revenue by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, we help with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

IVD Compliance Transition
Don’t let compliance challenges impede your progress. Velsera assists labs in navigating the FDA clearance process for both new assays and existing Laboratory Developed Tests (LDTs). We provide the necessary “dry-lab” component for approved products, along with optional tertiary reporting to enhance your workflow.

Companion diagnostic development & scaling
Don’t wait until the last minute to design and develop your Companion Diagnostic (CDx) – it literally is the rate limiting gate keeper to scaling your new therapy! Velsera can partner with you to design and develop your test by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, once live in the market, we can help labs with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

Real-world impact, trusted partnerships
“Interpreting large-scale, complex transcriptomics data is critically important for understanding the molecular and cellular basis of disease. We are confident that by working with the unique multi-cloud technology developed by Velsera and by benefiting from the depth and breadth of their expertise in bioinformatics and cloud computing, these solutions will assist us in maximizing the value of the data we generate.”

News
On January 22, 2026 | By Johanna Samuelsson
Read more

Blog
On July 15, 2025 | By Aaron Wenzel
Read more
News
On June 24, 2025 | By Brian Lassiter
Read more

News
On May 30, 2025 | By Brian Lassiter
Read more
Together, we can enhance research outcomes and improve patient care. Join us in creating a seamless flow of actionable insights that drive breakthroughs in healthcare and life sciences.