Velsera Appoints Jamie Coffin, PhD, as Chief Executive Officer
Read more

News
On January 22, 2026 | By Johanna Samuelsson
Read more

Blog
On July 15, 2025 | By Aaron Wenzel
Read more
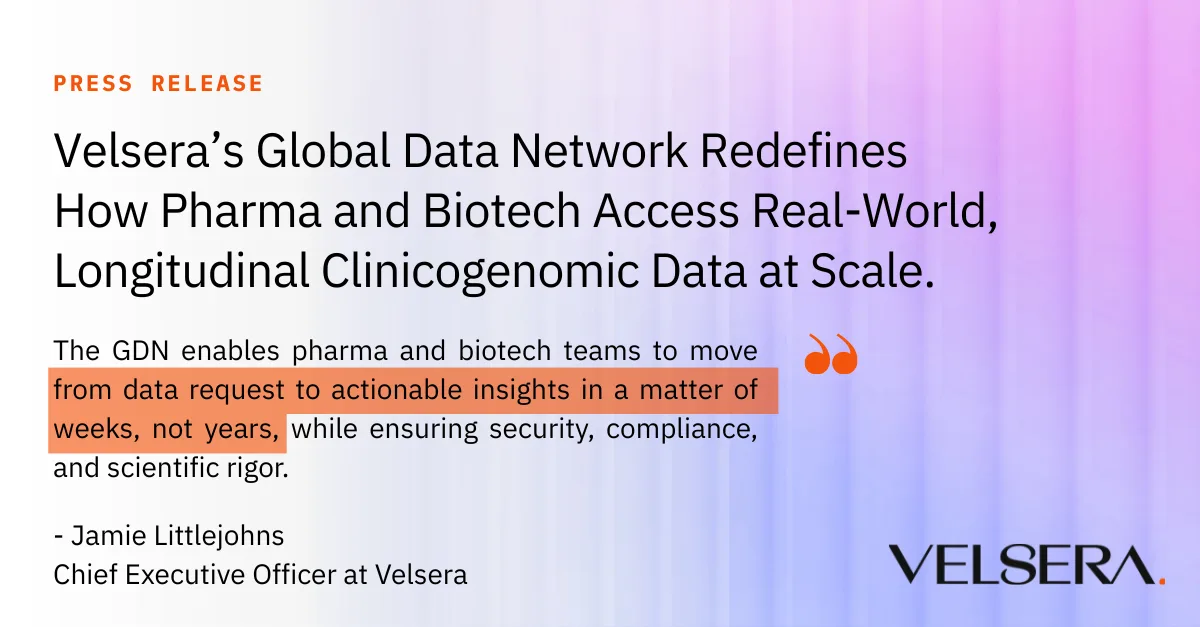
News
On June 24, 2025 | By Brian Lassiter
Read more

News
On May 30, 2025 | By Brian Lassiter
Read more

Blog
On April 29, 2025 | By Zelia Worman
Read more

Blog
On June 16, 2025 | By Indu Peruri
Read more
Blog
On May 19, 2025 | By Aaron Wenzel
Read more

Blog
On June 16, 2025 | By Liam Scott
Read more

Blog
On May 19, 2025 | By Michael Evenson
Read more

Blog
On April 2, 2025 | By Zelia Worman
Read more

Blog
On April 14, 2025 | By Laura Buttrum
Read more

Blog
On December 3, 2024 | By Julie Furt
Read more