Velsera Appoints Jamie Coffin, PhD, as Chief Executive Officer
Read more
Knowledgebase
Powering our Clinical Genomics Workspace decision support software, our knowledge base ensures your patients get the latest and greatest knowledge to support the optimum treatment decision.
With the continuous expansion of medical knowledge and new therapies, clinical labs face an overwhelming volume of genetic data that requires fast yet accurate interpretation. The Velsera Clinical Genomics Knowledgebase – a key feature of the Clinical Genomics Workspace – addresses this challenge by combining advanced technology with human expertise to build a knowledge base of clinical interpretations that enable laboratories to engage in the practice of medicine and assemble clinical content for each patient.
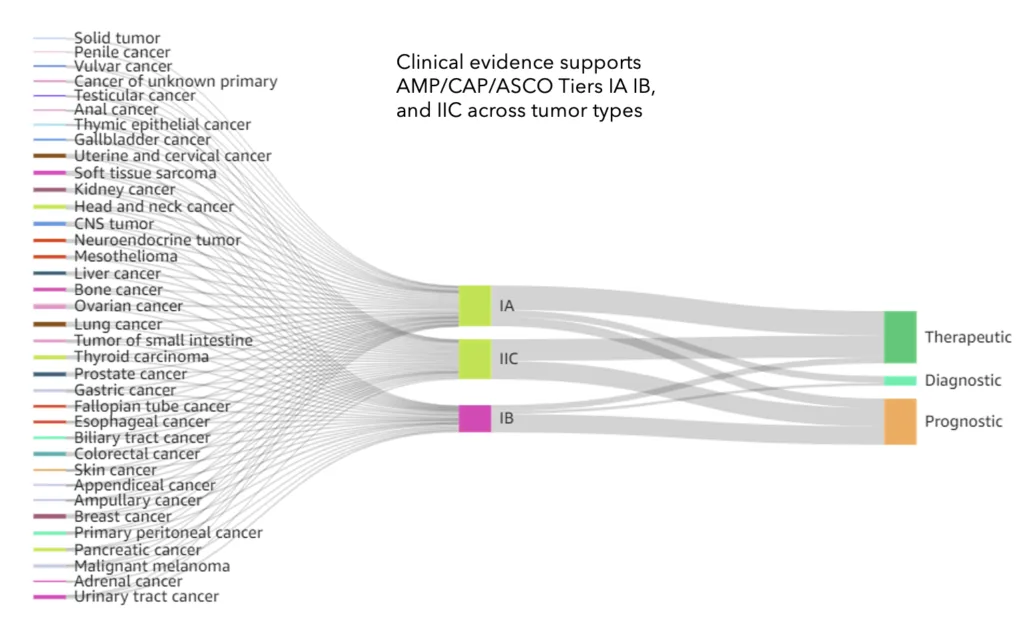
Why use Velsera’s Clinical Genomics Knowledge Base
With over 400,000 logical rules covering 1,250 somatic cancer genes, the Velsera Knowledgebase supports all major genomic variant types—including SNVs, TMB, MSI, indels, CNVs, fusions, and large structural variants—ensuring robust support for complex genomic testing scenarios.
The Velsera Clinical Genomics Knowledgebase draws from a wide array of sources to ensure precise variant interpretation, including:
This diverse dataset is continuously curated by Velsera’s team of PhD- and MS-level scientists, ensuring users can confidently interpret even the most complex genomic variants.
Velsera's expert biocurators continuously review and update the knowledgebase, ensuring users have access to the latest clinical insights. Each update is rigorously verified, following SOPs designed to meet ISO 13485, ensuring that every patient report is backed by trustworthy, up-to-date evidence. Our team of expert biocurators make monthly updates to our knowledge base which are pushed out to all of our CGW users.
Unlike simple variant lookups, Velsera’s Knowledgebase leverages a flexible metadata model and rules engine that allows for comprehensive content matching. Whether the variant has been previously described or not, the system applies logic based on gene-variant-disease patterns to generate meaningful interpretations. This future-proofs the platform for emerging biomarkers and evolving genomic technologies.
Who we help accelerate
Clinical Diagnostic Laboratories
Grow revenue by expanding your NGS business. Velsera helps diagnostic labs rapidly establish and grow new, profitable NGS revenue streams. With our support, we can help your lab get up-and-running with an existing on-market assay (20+ LDT & IVD options available), including support for validation, revenue cycle management, and our Clinical Genomics Workspace (CGW) software to aid clinical decision-making.

NGS Assay & Equipment Manufacturers
Have you got a promising diagnostic test in development? Or do you have novel equipment that you want to drive uptake of in the clinical setting? Velsera can partner with you to bring new, promising tests to market by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, we can help your future laboratory customers with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.
Biotech & Pharma
Don’t wait until the last minute to design and develop your Companion Diagnostic (CDx) – it literally is the rate limiting gate keeper to scaling your new therapy! Velsera can partner with you to design and develop your test by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, once live in the market, we can help labs with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

Solutions
Clinical Interpretation & Reporting
Grow revenue by expanding your NGS business. Velsera helps diagnostic labs rapidly establish and grow new, profitable NGS revenue streams. With our support, we can help your lab get up-and-running with an existing on-market assay (20+ LDT & IVD options available), including support for validation, revenue cycle management, and our Clinical Genomics Workspace (CGW) software to aid clinical decision-making.

NGS Assay Development & Expansion
Has your lab recognized an unmet need in the market? Velsera helps ambitious diagnostic labs generate revenue by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, we help with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

IVD Compliance Transition
Don’t let compliance challenges impede your progress. Velsera assists labs in navigating the FDA clearance process for both new assays and existing Laboratory Developed Tests (LDTs). We provide the necessary “dry-lab” component for approved products, along with optional tertiary reporting to enhance your workflow.

Companion diagnostic development & scaling
Don’t wait until the last minute to design and develop your Companion Diagnostic (CDx) – it literally is the rate limiting gate keeper to scaling your new therapy! Velsera can partner with you to design and develop your test by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, once live in the market, we can help labs with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

Real-world impact, trusted partnerships
“I knew that with the resources we had, there was no way to create something that would rival a system like the CGW. As we've set up reflex testing in various cancer types, we have been well supported to scale the massive undertaking.”

News
On January 22, 2026 | By Johanna Samuelsson
Read more

Blog
On July 15, 2025 | By Aaron Wenzel
Read more
News
On June 24, 2025 | By Brian Lassiter
Read more

News
On May 30, 2025 | By Brian Lassiter
Read more
Don’t wait