Velsera Appoints Jamie Coffin, PhD, as Chief Executive Officer
Read more
GRAF
Our award-winning reference genome tool.
Canonical Linear Reference genomes are now 20+ years old and do not account for all genomic variation. Read mapping against linear references leads to reference bias. This systemic blindness is a profound barrier to accurate genomic data analysis, especially in non-European populations.
GRAF for discovery
Run fast and accurate genomic analyses using genome graphs. GRAF represents genetic variation in a population by enhancing the standard reference. The canonical linear reference serves as the backbone while edges represent alternate sequences.

GRAF for pan genome-aware secondary NGS analysis
Velsera has pioneered graph reference-based secondary analysis technology since 2014. The technology and patent portfolio are now available as robust, stable, scalable, and deeply validated pipelines ready for deployment in pharma discovery environments. The draft human pan-genome reference presented by the HPRC1 has generated broad awareness of how to solve the reference bias problem. State-of-the-art graph and pan-genome-based sequence analysis is now becoming the standard.
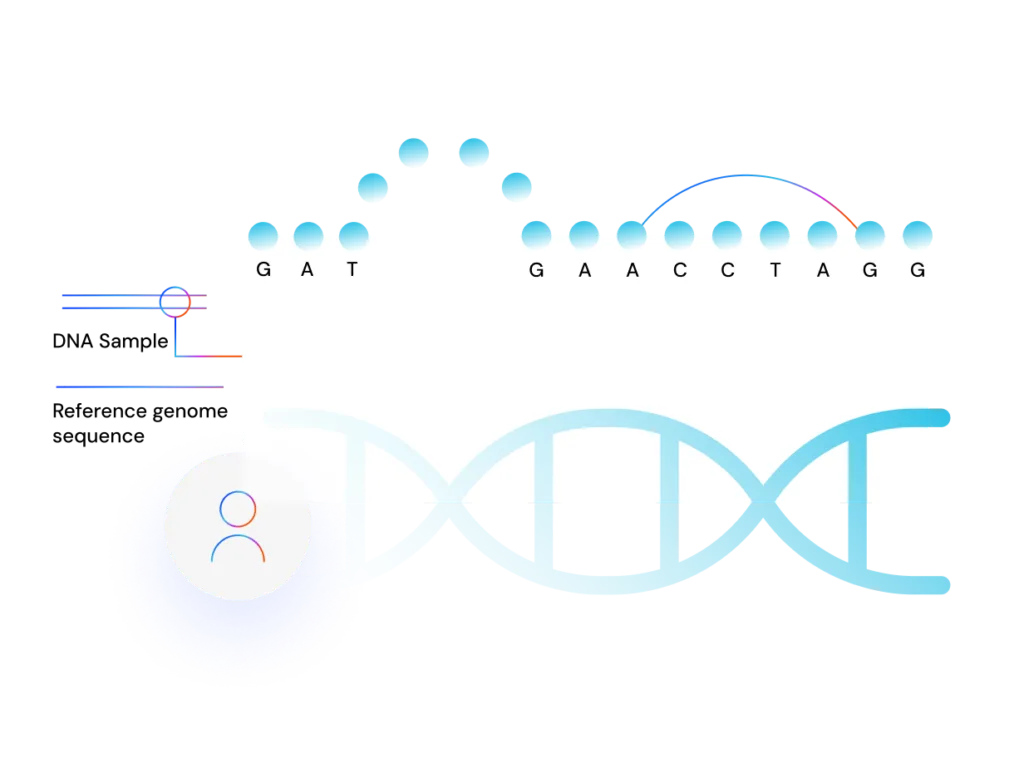
Why use GRAF
Known variants
The graph aligner aligns significantly more reads and accurately detects the variant as a homozygous insertion, while BWA erroneously detects it as heterozygous.
Identifying complex variants
Graph alignment facilitates the discovery of in-phase variants and other types of complex events.
Compound variants
A graph reference easily places variants within existing variants to facilitate alignment.
Across structural and point variants
Velsera’s GRAF solution provides data structures, algorithms, and graph references that effectively capture genomic diversity.
Who we help accelerate
Clinical Diagnostic Laboratories
Have you got a promising diagnostic test in development? Velsera can partner with you to bring new, promising tests to market by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines on Seven Bridges. Additionally, we can help your future laboratory customers with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

NGS Assay & Equipment Manufacturers
Have you got a promising diagnostic test in development? Or do you have novel equipment that you want to drive uptake of in the clinical setting? Velsera can partner with you to bring new, promising tests to market by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, we can help your future laboratory customers with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement – increasing the uptake from customers. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.
Biotech & Pharma
The volume of clinical-genomic data available to researchers is exploding. But without the right tools and advice it can be hard to cut through the noise and find the insights you need. Our best-in-class tools supported by our in-house Bioinformatics and Science teams can help you to do just this and accelerate your time to finding that next big breakthrough.

Non-profits & Government Agencies
Velsera’s tools are already widely in use across government, academic and commercial research. Our teams of in-house Scientists can help engage this community to make better use of your assets – either to further your research interests, drive new revenue streams, or both!

Solutions
Analysis & Bioinformatics
The volume of clinical-genomic data available to researchers is exploding. But without the right tools and advice it can be hard to cut through the noise and find the insights you need. Our best-in-class tools supported by our in-house Bioinformatics and Science teams can help you to do just this and accelerate your time to finding that next big breakthrough.

NGS Assay Development & Expansion
Has your lab recognized an unmet need in the market? Velsera helps ambitious diagnostic labs generate revenue by creating, integrating, hosting and/or deploying custom-developed NGS analysis pipelines. Additionally, we help with validation and regulatory support to ensure compliance, as well as Revenue Cycle Management support to enable reimbursement. Optionally, tertiary CGW reporting is available to create a fully integrated secondary & tertiary analysis package for your test.

Real-world impact, trusted partnerships
“Using standard computational tools, we are already finding a large number of novel variants of African and Native American ancestries within the Brazilian genomes. We want to use the graph-based approach in order to capture the full value of our genomes.”

News
On January 22, 2026 | By Johanna Samuelsson
Read more

Blog
On July 15, 2025 | By Aaron Wenzel
Read more
News
On June 24, 2025 | By Brian Lassiter
Read more

News
On May 30, 2025 | By Brian Lassiter
Read more